Institute for Protein Physics
Institute for Protein Physics
Research Theme Overview
(A) Development and application of molecular design methods aimed at improving proteinproperties.
(B) Development and application of data analysis methods related to protein physical properties,
| (A) Development and application of molecular design methods aimed at improving protein properties. | |||||||
|---|---|---|---|---|---|---|---|
|
 |
||
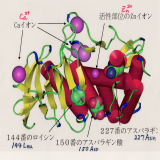
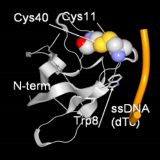
| (A.3) Molecular design for higher affinity Molecular designs are performed to have higher affinity by utilizing conformational changes and multivalent bindings. |
| (B) Ddevelopment and application of data analysis methods related to protein physical properties. | |||||||
|---|---|---|---|---|---|---|---|
|
 |
||
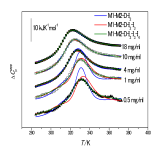
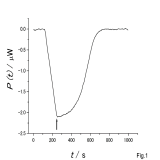
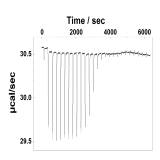
| (B.3) Evaluation of binding affinity Based on ITC (isothermal titration calorimetry) and various spectroscopic data, we use equilibrium models to evaluate binding and dissociation constants. |