Institute for Protein Physics
Topics
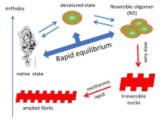
(T.1) Reversible oligomers of denatured protein molecules
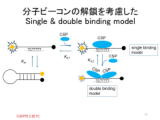
(T.2) Binding mechanism of cold shock protein (CSP) tosingle-stranded nucleic acid
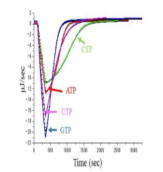
(T.3) Evaluation of ATPase activity and inhibition byisothermal titration calorimetry (ITC)
(T.4) 40th anniversary of the start of DSC of proteins
(T.5) A serial article on "Calorimetry of Protein Molecules" has started in Netsu Sokutei
(T.6) 40th Anniversary of the Development of DoubleDeconvolution (DDCL) Method
(T.7) 25th Anniversary of the Publication of the FirstPaper on Enzyme Activity Assessment Using IsothermalTitration Calorimetry (ITC)
 |
(T.1) Reversible oligomers of denatured protein molecules | |
| Denatured protein molecules are generally prone to association, and are often thought to irreversibly form large aggregates that become insoluble. The main reason why a boiled egg does not return to its raw state even when the temperature is lowered is because of the irreversibility of this aggregation. However, recent researches have reported examples of various proteins in which a few denatured protein molecules reversibly associate to form a single thermodynamic state that is stable in equilibrium. Experimental results have also been reported that indicate the possibility that these aggregates may serve as nucleus precursors for the formation of amyloid, a type of irreversible aggregate. We are continuing our research into the reversible aggregates formed by such denatured proteins, calling them ROs (reversible oligomers). [References] Latest papers, latest reviews |
 |
(T.2) Binding mechanism of cold shock protein (CSP) to single-stranded nucleic acid | |
| Cold shock proteins (CSPs) bind strongly to single-stranded nucleic acids and have important function of controlling the three-dimensional structure of nucleic acid in cells. Isothermal titration calorimetry (ITC) is used to analyze the binding ability, but due to sensitivity issues, it is usually performed at a concentration of about μM. These data are analyzed using a simple one-to-one binding model (single binding model), but there was a problem that the van't Hoff enthalpy calculated from the temperature dependence of the binding constant did not match the calorimetric enthalpy directly measured by ITC. By using molecular beacons (MBs) designed with controlled stability, we succeeded in evaluating the binding ability at high sensitivity both at the nM level and at the μM level by fluorescence measurement. As a result, it was suggested that the binding constant when one molecule binds to a base sequence to which CSP specifically binds is one order of magnitude larger than the value evaluated by ITC, and that at a concentration of μM, the binding constant is underestimated due to cooperative binding of two CSP molecules to single-stranded nucleic acid. (the 60th Anniversary Japanese Conference on Calorimetry and Thermal Analysis, Kyoto Prefectural University, September 2024)Abstract |
 |
(T.3) Evaluation of ATPase activity and inhibition by isothermal titration calorimetry (ITC) | |
| IIn this study, the entire time course of the hydrolysis reaction of ATP etc. by the stable helicase domain derived from Tomato Mosaic Virus was observed by isothermal titration calorimetry (ITC). Helicase is an enzyme that has the function of unwinding the double helix formed by nucleic acids such as DNA into single strands using the energy of ATP hydrolysis. Analysis of the entire time course by changing the initial concentration of the substrate showed the dependence of the apparent Michaelis constant on the initial substrate concentration, and competitive inhibition by the product (ADP etc.) was clearly demonstrated. By comparing the true Michaelis constant with the inhibition constant, it was found that the enzyme has a 17-fold higher affinity for ATP than for ADP. In addition, inhibitors of this enzyme were screened using this method. (The results of this study have been accepted by Netsu Sokutei, the Journal of Japan Society of Calorimetry and Thermal Analysis, with Professor Etsuko Katoh of Toyo University as the first author.) [article] |
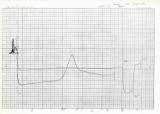 |
(T.4) 40th anniversary of the start of DSC of proteins | |
| It has been exactly 40 years since the first DSC measurement was performed on January 24, 1985 at the Institute of Fiber and Polymer Materials, Agency of Industrial Science and Technology (now National Institute of Advanced Industrial Science and Technology) in Tsukuba. I would like to express my deep gratitude to Dr. Hatsuho Uedaira (then at the Institute of Fiber and Polymer Materials) and my supervisor at the time, Professor Akiyoshi Wada, for their guidance in the measurement. The chart on the left is the measurement taken at that time. The thermal transition of the porcine enzyme protein pepsinogen at pH 8.3 was measured at a heating rate of 1 K/min using a high-precision adiabatic differential scanning calorimeter, DASM-1M, made in the Soviet Union (now Russia), the only one in Japan at the time. A broad endothermic peak was observed on the high-temperature side of the endothermic peak associated with the thermal transition. [See Fig. 6 in the published paper] |
 |
(T.5)A serial article on "Calorimetry of Protein Molecules" has started in Netsu Sokutei" | |
| I am currently running a four-part tutorial series entitled "Calorimetry Measurement of Protein Molecules" in the journal "Netsu Sokutei" of the Japan Society of Calorimetry and Thermal Analysis, starting from the April 2025 issue. The first article is now published. To request a reprint, please contact "kidokoro@proteinphys.org". The first part is as follows, explaining and introducing the characteristics of calorimetry of protein molecules, the measurement principles, and the shared use of measurement equipment. 1. Introduction 2. Characteristics of protein molecules as measurement targets 3. Principles of calorimetric measurement of protein molecules 4. Isothermal titration calorimetry (ITC) 5. Shared use of calorimeter 「1st article} The second part covers the following topics, and describes methods for measuring and analyzing transitions between various thermodynamic states, focusing on conformational changes and stability of protein molecules. 1. Introduction 2. Various thermodynamic states exhibited by protein molecules 3. Observation of structural transitions with reversible process 4. DSC of irreversible conformational transitions [2nd article] The third article focuses on calorimetry of protein-ligand binding, covering the following topics. In particular, we discuss the handling of heat from buffers used to maintain a constant pH, analysis using the simplest one-to-one model, and application to more complex systems. 1. Introduction 2. Hydrogen Ions: A Ligand Common to Protein Molecules 3. Dissociation Enthalpy of Buffer Hydrogen Ions 4. Enzyme-Inhibitor Binding Evaluation 5. Binding Evaluation in More Complex Systems [3rd article] In the fourth and final article, I explain the advantages of calorimetry in assessing the catalytic activity of protein molecules based on the well-known Michaelis-Menten mechanism. 1. Introduction 2. Steady-state enzyme reactions and the Michaelis-Menten equation 3. Advantages of calorimetry in observing enzyme reactions 4. Time course measurements of enzyme reactions |
On August 27, 1985, we submitted a paper to Biopolymers, which was accepted approximately one year later on August 25, 1986, and published in 1987. This paper presented a statistical mechanical basis for analyzing the heat capacity (DSC data) of proteins and other substances. It also demonstrated that, using the same procedure as the then-prevailing two-state analysis (estimating the heat capacity function for two states, low and high, from experimental data), it is possible to evaluate the mole fraction of each state and the enthalpy function in the presence of intermediate states, without assuming the number of states. While this method was developed for systems in which the equilibrium does not change with protein concentration, our second paperpublished approximately one year later showed that it can be extended to systems involving self-dissociation and association processes. This method has been applied to a variety of systems over the past 40 years, and we would like to provide technical support to further broaden its use.
「Next page]